Latest Drugwonks' Blog
One of the myths perpetuated during the debate over the Affordable Care Act (and nowhere more so than in the Michael Moore movie “SiCKO”) was that it would provide “free” health care “like in Europe.”
Such myths die hard – and especially in Vermont.
The Wall Street Journal writes:
Last week, in a reversal that deserves more attention, Democratic Governor Peter Shumlin announced that Vermont would no longer create America’s first statewide single-payer health system. Vermont was seeking a waiver from the Affordable Care Act to abolish what’s left of the nominally private insurance market by 2017, but Mr. Shumlin’s budget gremlins concluded the plan was too expensive and would damage the state economy … The state accountants estimated that his plan required an 11.5% tax on worker payroll, with no exceptions. Individuals, meanwhile, would have paid as much as 9.5% of earnings, which would have applied to everyone making more than four times the poverty level, or $102,220 for a family of four—hardly the 1%. The full $2.59 billion in necessary funding would roughly double current state revenues (about $2.85 billion today).
(The full Wall Street Journal editorial can be found here.)
In other words, there is no such thing as “free” government-supplied health care.
This shouldn’t have come as a surprise. In Canada, while the percentage of taxes used to provide health care varies, it’s estimated that about 22% of taxes collected go into the health system and several provinces, including Quebec, Ontario, Alberta, and British Columbia also charge additional premiums. Citizens in the U.K. pay 11% of each pound they make in weekly income, between £100 and £670 for the NHS,
And what can’t be overlooked is that price controls equals choice controls.
When it comes to the Affordable Care Act, patients can access any medicine they need -- as long as it's on the exchange formulary. Sure, the ACA limits the degree to which insurers can charge higher premiums for sicker patients, but ObamaCare plans found a way around these rules: impose higher out-of-pocket costs for all or most specialty drugs. High co-pays effectively remove choice from the system for many patients.
The breakdown of Silver plans (the most popular category) is particularly revealing. In seven classes of drugs for conditions from cancer to bipolar disorder, more than a fifth of these plans require patients to shoulder 40 percent of the medicine’s cost. And 60 percent of Silver plans place all drugs for illnesses like multiple sclerosis and rheumatoid arthritis in the “formulary tier” with the highest level of cost-sharing.
Nearly every Silver plan across the country, in fact, puts at least one class of drug exclusively in the top cost-sharing tier. In effect, this leaves patients with a given condition — whether HIV or Crohn’s disease — without a single affordable treatment option. Silver is the new Black.
Referring to the Model T, Henry Ford famously said, "Any customer can have a car painted any color that he wants so long as it is black.” That worked out fine – until there was competition. Choice is the great emancipator. The same is true when it comes to healthcare – and a lot more important.
Improving the Affordable Care Act to help the chronically ill
By Larry Hausner
December 20, 2014
The Hill
This year, one in three Americans chose to forego medical care for themselves or a family member because of cost concerns, according to a new Gallup poll.
The Affordable Care Act was supposed to prevent those situations and extend reasonably priced health care to everyone. But insurance companies have been exploiting loopholes in the law to avoid this obligation.
Fortunately, the Centers for Medicare and Medicaid Services -- the federal agency that regulates health insurance -- recently proposed a new rule that will close such loopholes and strengthen patient protections.
As the organization finalizes its regulations, it has come under pressure from insurance industry lobbyists to water them down. The CMS should resist these efforts and install genuine reforms that will improve health coverage for vulnerable Americans.
These new regulations take aim at one of the biggest shortcomings of the Affordable Care Act -- unreasonably high patient cost-sharing. The healthcare law caps out-of-pocket expenses at $6,350 for individual plans and $12,700 for family plans. After an insured person or family hits the cap, the insurance company must pay the rest of the treatment costs.
However, insurers have found a way around that cap by forcing families to pay all the way up to that $12,700 threshold even when only a single household member is sick. The new CMS regulations would prohibit insurance companies from charging more than $6,350 for treating any one patient.
This change protects families and furthers the original principle of the ACA: no one should go bankrupt because of disease.
Insurance companies have also been burdening patients who have qualified for so-called "cost-sharing reduction" plans. These plans feature lower out-of-pocket maximums than standard plans, but are available only to patients earning less than 250 percent of the federal poverty line.
Many of these plans include absurdly high co-insurance rates. A new report by Milliman, a major consulting firm, finds that 22 percent of cost-sharing reduction plans feature co-insurance rates of 50 percent or more for certain specialty drugs. In other words, insurance companies are forcing some of the poorest patients in the country to pay the lion's share of their prescription drug costs.
The new CMS regulations will require insurers to summarize the coverage and benefits for these cost-sharing reduction plans and present that information clearly to customers. That's a welcome move, but the agency could go further by prohibiting insurers from charging poor patients co-insurance at all.
The new rule also addresses the problem of rampant insurer discrimination against some of the most vulnerable Americans -- those suffering from serious diseases like diabetes, cancer, and multiple sclerosis.
A study by Avalere Health, a prominent healthcare advisory company, discovered that an alarming 86 percent of the most common insurance plans sold through the new state-level exchanges placed all medications for at least one drug type in the highest cost-sharing tier.
In practice, this means that, say, a diabetes patient might be hit with extremely high out-of-pocket expenses when she goes to fill a prescription for key treatments. The Avalere study found that many exchange plans require 30 percent or more "co-insurance" for all diabetes drugs. Co-insurance forces patients to bear a set percentage of the final drug bill, regardless of how big it is.
In its proposed rule, CMS clarifies that this practice is a form of discrimination against patients. This call-out is commendable but insufficient. The agency should also prohibit companies from pricing all drugs of a particular type out of reach for average patients.
The proposed CMS rule will stop insurers from another method of discrimination -- changing a plan's cost-sharing or benefit structure halfway through the year, after consumers are already locked-in. CMS should also make sure insurers aren’t allowed to drop drug coverage mid-year.
Finally, the agency's reforms will address a general lack of transparency in insurance plans. Insurers will be required to publish a complete list of covered drugs and their relevant cost-sharing tiers. This change will help patients better understand their plan's coverage and benefits. CMS could further improve transparency by mandating that insurers provide specific cost-sharing information for each drug.
CMS deserves praise for its newly proposed rule. It will help combat unreasonable cost-sharing arrangements, patient discrimination, and a lack of transparency. With just a few more added patient protections, the agency can ensure that quality health insurance is truly affordable for all.
Hausner is the former CEO of the American Diabetes Association.
What do we fear more: guns being used in murder or suicide? I think the answer is obvious. And it shapes our behavior, our culture and public policy. But what is the real risk?
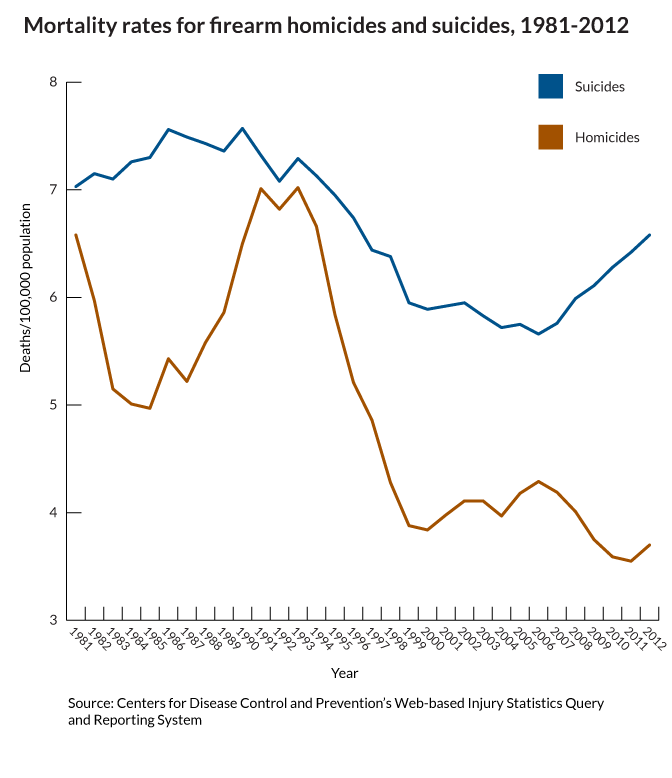
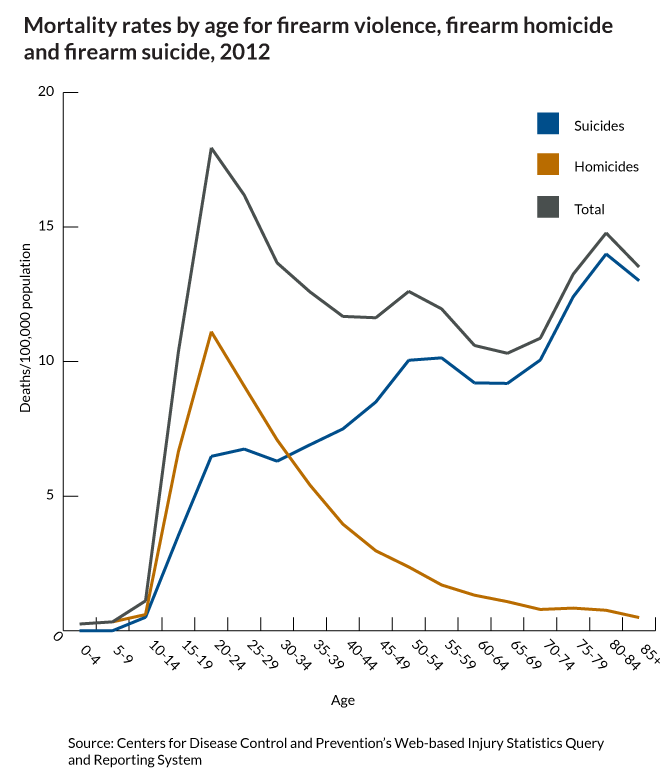
This gun violence fact is only "startling" because of how the media reinforces the fear of the unseen, outside danger...
December 18, 2014, 02:00 pm
Closing the gap between testing and treatment
The Hill
By John Morrissey
I have just returned from San Francisco and a meeting of more than 20-thousand blood cancer experts at a global hematology conference. I was there as part of a new mission to rapidly-but-responsibly turn the data from these medical meetings into treatments.
A new alliance CLOSING-THE-GAP-NOW held a historic first conference at the University of Minnesota in mid-November. Our goal is to streamline medical oversight. I know it can be done. Under Presidents Nixon and Ford I worked to replace rules that delayed and discouraged advances in transportation.
ADVERTISEMENT
We are ready to the same for modern medicine. It is a moral imperative.
Consider. Today when a patient is prescribed a so-called new cancer treatment, it is actually the product of testing that left the laboratory as much as ten years before. That delay, the years and expense of testing, is the force driving up the costs of new therapies while denying them to patients.
The result? Tragic stories like the recent, very public case of Brittany Maynard. Diagnosed with a brain tumor and given but a few months to live, she raised the issue of the right to die. Her story really should have us thinking about what patients need to survive.
I know first hand how these lies of omission can deny hope and atrophy will to live.
I received the message that nothing can help in March 2009 when my wife contracted pancreatic cancer. Six weeks after I lost my wife, I heard that same message in October 2009 when my daughter was diagnosed with brain cancer.
The problem is, what we were told was just not the case.
In fact, there were a number of promising mainstream medical treatments in various stages of development. I found them for my family. Those innovative treatments gave us a little more time together as a family, and time is precious when it is running out.
How can we offer these same opportunities to every cancer patient?
Let’s understand that the current system is a product of the sixties. But there could be a better, faster and safer path.
Let’s harness the real power of new science, of big data, of the specificity of genome sequencing. Let’s re-imagine a review process in which each patient would get the best option and the most advanced care. Then, using computing tools with Google-like power we can capture and share all the data from their experiences. Every patient could in-effect be part of a huge virtual clinical trial while getting the best personal care.
A deluge of new information could begin to be available in ten weeks instead of ten years. Patients would not be kept waiting when they simply have no time to wait.
Thinking of tragic stories like Brittany Maynard’s, it’s long past time to appreciate the value of medical innovation and how we can use it to value each and every human life by updating the procedures and regulations that are supposed to enhance care, not interfere.
Morrissey is chair of the Scientific Advisory Board at the Richard M Schulze Family Foundation.
I’ve just returned from a visit to the UAE and Kuwait where I had the privilege of speaking with medicines regulators and pricing authority representatives from those two nations.
The topic of conversation was the value of innovation – and the urgency of rewarding it through timely approvals and appropriate pricing and reimbursement.
Our conversations ranged over many topics but focused specifically on a few key points:
* When patients have access to more effective medications, their overall health improves, even as their overall medical expenses go down. That, in turn, reduces national health-care spending and boosts the economy. Value must be measured in patient outcomes.
* Healthcare innovation saves lives, saves money, promotes economic growth, and provides hope for hundreds of millions of people (both patients and care-givers) in the United States and around the world.
* If we do not support the development of new medicines through timely licensing and fair pricing, innovation will be stopped in its tracks – and that is not an acceptable public health outcome.
* Regulators can be partners in innovation three ways: Through robust oversight, through active collaboration, and, most importantly, by being an innovation enabler.
We spent a lot of the conversation discussing the important differences between value and pricing – and that while both are important, it is value that’s the higher priority since driving patient outcomes is the higher calling (and better long-term economic investment).
After all, as Yale economist William Nordhaus has written, "The social productivity of health care spending might be many times that of other spending.”
Insha'Allah.
Emanuel noted he 'almost came to blows with the editor of the Atlantic" because he hated the title of the article. He made three important points that were not fully discussed in his article:
1. We should measure life in terms of how meaningful it is, not how long it is.
2. We should focus less on paying for end of life care and more on improving well-being in expectant mothers and adolescents.
3. We should measure meaning in terms of the capacity to create, to share and enjoy the company of others.
Ironically, the title of the article generated a lot of controversy, most likely by people who did not read his piece and used the title confirm their belief that Zeke wants to kill everyone over the age of 75. The title of the article could have been: "I Want To Live Better, Not Just Longer" but it wouldn't have created the same stir..
"SEPTA has filed a class action suit at the U.S. District Court for the Eastern District of SEPTA logoPensylvania against Gilead Sciences, Inc. related to the sale and pricing of its Hepatitis-C drug, Sovaldi.
Sovaldi is the first drug approved by the Food and Drug Administration for certain types of Hepatitis-C infections that does not need to be injected. It can reportedly cure about 90 percent of patients with the most common form of Hepatitis-C in three to six months, and can do so with relatively minor side effects compared to earlier available treatments.
Gilead has been selling a 12-week regimen of Sovaldi in the United States for approximately $84,000, or $1,000 per pill. This is significantly more than the original price projection for Sovaldi, and in sharp contrast to the prices at which the drug is being made available in other countries, the complaint says.
Gilead recently announced its intention to make Sovaldi available in 91 developing countries at deeply discounted prices, and the drug is reportedly available in Egypt for 99 percent below the U.S. price.
While there are some orphan drugs that are similarly expensive, they are typically limited to rare conditions that affect only a very small patient population. In those instances, charging high prices may be necessary to recoup amounts invested in research and development.
In the case of Sovaldi, however, there are between 2.7 and 5.2 million people in the United States infected with Hepatitis-C, and 185 million people worldwide. The complaint alleges that, if left unchecked, Gilead’s exorbitant pricing scheme has the potential to bankrupt segments of the U.S. healthcare system."
Ok, let's go through the facts again..
The total lifetime cost of treating every HCV patient absent such innovations would be $360 billion. That does not include an estimated $400 billion loss in productivity and $3 trillion in health value lost because of premature mortality.
From this perspective it is clear that new medicines almost always reduce the cost of living longer and healthier life and increase the value of such improvements. Further, it is clear that the price of new drugs, setting aside the obvious need for faster and smarter drug development costs, reflect the high percentage of social value generated by medical innovation. Assuming the $240 billion cost goes right to innovators, more than 90% of the value of the product ($360 bill + $400 bill. + $3 trillion = $3.76 trillion) goes to society.
Bioequivalence is going mainstream.
Ever since the FDA’s Pharmaceutical Science and Clinical Pharmacology Advisory Committee concluded in April of 2010 (in an 11-2 vote) that current bioequivalence standards are not sufficient for narrow therapeutic index (NTI) drugs, there’s been a growing focus and understanding on the fact that less variability equals better predictability.
(Narrow therapeutic index, per the FDA, means that, “small changes in blood concentration have the potential to result in serious therapeutic failures and/or serious adverse drug reactions.”)
Currently, the “sameness” of a brand product and a generic version is evaluated based on a two-treatment crossover study to prove bioequivalence, the aim being to show that the 90 percent confidence intervals of the geometric mean test/reference ratios for both maximum plasma concentration and the area under the plasma concentration-time curve fall within a range of 80 percent to 125 percent.
(“Equivalent” doesn’t mean the same thing as “identical.”)
“All men are created equivalent” just doesn’t have the same ring, does it?
This is an urgent public health issue – and it’s got momentum.
It’s too simplistic to call these “quality” problems. There’s a range from sub-standard Active Pharmaceutical Ingredient (API) and manufacturing issues, to excipient changes (excipients are the substances other than the pharmacologically active drug contained in a pill) and, most importantly, bioequivalence and bioavailability standards.
“We are losing control over what people are swallowing,” said Dr. Harry Lever, a cardiologist at the Cleveland Clinic who is trying to raise awareness of the matter among U.S. lawmakers. “Now, when a patient comes in who is not doing well, the first thing I do is look at their drugs and find out who makes it.”
Bioequivalence does not always equal therapeutic equivalence – and that’s especially true for Toprol XL. Recently two large Indian manufacturers, Wockhardt and Dr. Reddy’s Laboratories, have announced recalls over the last two months totaling more than 100,000 bottles of Toprol XL because their products were not dissolving properly — therefore probably not working as they should. The drug is a beta blocker that treats high blood pressure and heart ailments.
The issue of bioequivalence and therapeutic substitution will be at or near the top of the budget agenda in state capitols across the United States. NTI generic drugs, biosimilars and non-biologic complex drugs (NBCDs) are a medical option, but as every state in the union attempts to tighten its budget, requiring patients to use these follow-on products is an enticing, but incorrect and dangerous policy option. Placing short-term budgetary considerations before long-term patient well-being is pennywise and pound foolish, and is deleterious to both the public purse and public health. As an article in the Journal of Infection so aptly stated, “Nothing is more expensive than treatment failure.”
FDA’s recent draft guidances on bioequivalence for both generic and innovator products, as well as the move towards independent labeling for generic products are additional steps the agency has recently taken to address the issue of drug quality beyond safety and efficacy. And the implications for NTI generics, biosimilars and NBCDs is obvious.
(Something else to consider is for the FDA to report bioequivalence data in labels.)
The bioequivalence issue is clearly rising up the list of FDA priorities. Consider the agency’s action last week when it informed Mallinckrodt its methylphenidate hydrochloride tablets might not be therapeutically equivalent to Concerta, which is made by Johnson & Johnson ’s Janssen Pharmaceuticals Inc.
The FDA, in a statement, said the drug produced by Mallinckrodt may deliver the drug at a slower rate than another generic version of Concerta. As a result of its analysis, the FDA said the Mallinckrodt products are still approved and are eligible to be prescribed, but it no longer recommends them as an automatic substitution for Concerta.
But there’s a serious process issue that cannot be ignored. Mallinckrodt said the FDA informed the company that the change was based on new draft guidance for determining equivalency between the drugs that was published on November 6th – but the guidance has an open comment period that runs through January 5th, 2015. The FDA said this change was based on the application of its new Draft Guidance for determining bioequivalence of methylphenidate hydrochloride products just published on November 6, 2014. Although the Draft Guidance has an open comment period through January 5, 2015, the agency nevertheless confirmed that this change would be reflected on November 13, 2014 in the on-line Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations.
That may or may not be the proper public health decision – but it certainly doesn’t seem fair relative to thoughtful public comment. Not surprisingly, Mallinckrodt is suing the U.S. Food and Drug Administration for “unlawful” reclassification.
This is not a trivial issue. Regulatory predictability is at the foundation of the problem – and is the keystone of the solution. Minus sound and timely guidance, companies will think twice about whether or not to proceed with generic copies of medicines with a narrower therapeutic index. And that equates to less competition – which results in higher prices. This is as true for NTI medicines as it is for biosimilars. The good news is that guidances exist. The same is not true (at least not yet) for NBCDs. That should be next on the agenda – especially as it relates to A/B ratings. After all, if there are going to four categories of biosimilars, each with its significant impact on therapeutic interchangability, shouldn’t there be a commensurate finesse for similarly complex non-biologics approved under the Hatch-Waxman generic approval process?
As my friend and colleague, Dr. Scott Gottlieb has written:
The complex drugs fall in a regulatory gap. FDA has tried to retrofit the “Hatch Waxman” generic drug law and policies that govern approval of small molecule drugs to these complex drugs, with sometimes troubling results. Regardless of the decision FDA makes with Copaxone, it remains clear that Congress and FDA alike need to re-examine the regulatory process when it comes to these intricate drugs.
The problem is that FDA has refused to define these complex drugs as distinct from normal, small molecule medicines. That has forced the agency to rely on less information in approving these complex copies than it probably would like. The agency’s desire to try and squeeze these complex drugs through its existing generic law approval pathway may have as much to do with political expediency as with good science. FDA is probably well aware that getting Congress to define a distinct category for these medicines, and give FDA proper tools, could be a heavy political lift. So FDA is doing what it often does: trying to massage its existing authorities and regulatory practices to fit novel challenges. But at what cost?
The good news is that the FDA recognizes the need for more specific, regular, and predictable standards. That’s precisely why the agency has asked industry to identify drug products that require explicit bioequivalence testing guidances.
Robert Lionberger, head of the FDA’s Office of Research and Standards, said the agency’s objective is to develop a product-specific BE guidance before a manufacturer files an ANDA for the drug in question.
That bad news, per a report in Drug Industry Daily, is that the agency has more than 1,200 product-specific guidances posted to aid ANDAs, leaving hundreds of potential generic targets without an FDA-sanctioned roadmap for development. Their absence has complicated the process of submitting successful ANDAs.
Is the NBCD pathway, for example, something that should be considered as part of the pending 21st Century Cures legislation?
We are increasingly living in an n-of-1 world. Small is the new Big. We must think differently about bioequivalence on the front end and pharmacovigilance on the back-end. While we must continue to capture adverse event data, we must also strive to capture Substandard Pharmaceutical Events (SPEs). SPEs occur when a product does not perform as expected—perhaps because of API or excipient issues – or too broad of a bioequivalence range and issues relating to therapeutic interchangeability.
When it comes to 21st-century pharmacovigilance, we have to both broaden and narrow our views about bioequivalence to the patient level. Outcomes is so much more than a value-based reimbursement issue.
There is a growing awareness that science of attacking cancer is reaching a critical mass and is producing a truly transformational approach to treatment: one that re-engineers our natural immune responses to do the job of not just killing cancer cells but teaches the remaining ones to start become healthy.
There is a growing awareness that the way insurers have shifted cost to patients is discriminatory and that the claim that it’s a reaction to high prices is bogus.
There is a growing recognition that the value new medicines yield must be measured in terms of every month of every life gained. More people are alive today because each era of treatments keeps people alive longer for the next generation of therapies that improve and lengthen life even more.
And there is a growing recognition that the pace of progress is too damn slow, too dependent on outdate scientific methods that require people dying of disease to be randomized to different treatments or different combinations of treatments in ways that have little to do with their unique and increasingly accessible biological differences.
There is an increasing emphasis on improving the quality of life and the fact being treated for cancer does not mean being treated like a victim.
And finally, people are increasingly aware that by sharing information more quickly about the real world experience with medicines is the most scientific and efficient way to cure cancer.
I am honored to be part of this courageous community of advocates, patients, scientists and biopharmaceutical companies that express their awareness with growing discontent with the status quo. As Thomas Edison said: “Discontent is the first necessity of progress.”
At the same time that regulators in France, Germany, Belgium and Luxembourg are suspending the marketing approval of 25 generic drugs due to concerns over the quality of data from clinical trials conducted by India's GVK Biosciences, another Indian firm, Zydus Cadila, has launched a biosimilar of Adalimumab (an anti-TNF-α monoclonal antibody, which is approved in many countries for the treatment of inflammatory diseases, including rheumatoid arthritis, plaque psoriasis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn's disease and ulcerative colitis.)
Bad timing and, perhaps, bad science.
The quality of Indian pharmaceuticals has come under fire this year, with regulators in Europe and the United States citing problems ranging from data manipulation to sanitation and banning the import of certain products from several firms. And, it should be noted, these violations are for generic drugs, not biosimilars.
French and German regulators are investigating drug approvals based on clinical trials meant to show that these generic drugs were equivalent to the original branded versions conducted by GVK Bio between 2008 and 2014.
A Zydus Cadila spokesman commented that, “This therapy will offer a new lease of life to millions in India who have not had access to this therapy so far.” But the reality is that their product is more likely to be aggressively marketed for export.
Most importantly is the question of quality – or lack thereof. As company’s ranging from biotech innovator Amgen to multinational generics manufacturer Sandoz struggle to fulfill the clinical requirements for an Adalimumab biosimilar, two obvious questions arise -- (1) What does Zydus Cadila know that large multinational manufacturers don’t; and (2) What do Indian regulators know that their counterparts in the US and the EU are struggling to understand?
To not aggressively ask these questions is to bury one’s head in the sand.
And here’s an even tougher question -- In order to boost pharmaceutical exports, are Indian regulators willing to license substandard products?
According to a May 2012 article in The Lancet,
“To say that India's drug regulatory authority, the Central Drugs Standard Control Organisation (CDSCO)-whose remit includes new drug approval, licensing of manufacturing facilities, and regulation of drug trials-is not fit for purpose seems a gross understatement.”
“If I have to follow U.S. standards in inspecting facilities supplying to the Indian market,” G. N. Singh, India’s top drug regulator, said in an interview with an Indian newspaper, “we will have to shut almost all of those.”
Does that mean US standards are too high or that Indian ones are too low? Well, where you stand depends on where you sit. And if you’re sober and sitting up straight, the answer is obvious.
According to Dilip Shah, Secretary General of the Indian Pharmaceutical Alliance, some Indian manufacturers “doctor their data.” The solution? “Regulators should have regular liaison with manufacturers in India and China to explain to them how GMP works and that they don’t have to cheat.”
Feel better now?




